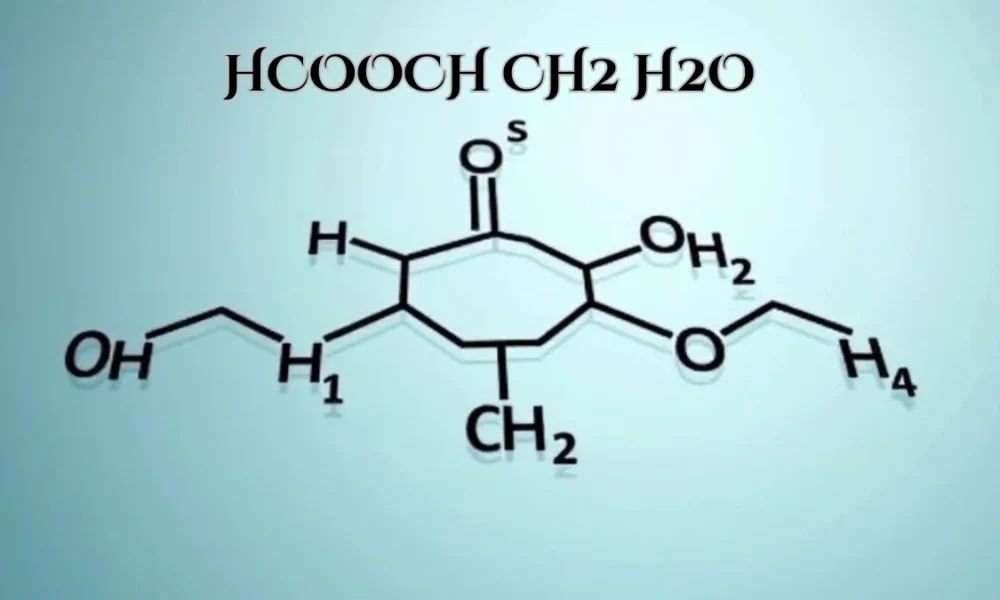
In the intricate world of organic chemistry, certain molecular structures stand out due to their unique compositions and versatile applications. One such structure is HCOOCH CH₂ H₂O, a compound that, at first glance, may seem complex but holds significant importance across various scientific and industrial domains. This article delves into the composition, properties, reactions, and applications of HCOOCH CH₂ H₂O, shedding light on its multifaceted role in modern chemistry.
Dissecting the Molecular Structure of HCOOCH CH₂ H₂O
The molecular structure of HCOOCH CH₂ H₂O may seem complex at first, but understanding its components reveals a simple yet fascinating composition that plays a vital role in various chemical processes. Let’s break down its components:
-
HCOOCH: This part denotes a formate ester group, formed when formic acid (HCOOH) reacts with methanol (CH₃OH). Formate esters, such as the one found in HCOOCH CH₂ H₂O, are notable for their reactivity. These esters are frequently used in organic synthesis, where their ability to act as intermediate compounds allows the creation of various chemicals and materials.
-
CH₂: The methylene group (CH₂) is a simple carbon-hydrogen unit, often referred to as a linker in organic molecules. This two-carbon unit facilitates the formation of more complex structures by connecting various molecular groups. The CH₂ group is vital in many biochemical and industrial reactions, enabling greater versatility in the formation of larger molecules.
-
H₂O: Water plays an essential role in chemistry, often facilitating or driving chemical reactions through hydration. In this molecule, water is integral to the compound’s properties, aiding in processes such as hydrolysis and ensuring the compound’s stability in different environments.
By combining these components, HCOOCH CH₂ H₂O demonstrates the power of ester and alcohol interactions, resulting in a compound with properties that are valuable for both laboratory and industrial applications.
Chemical Reactions Involving HCOOCH CH₂ H₂O
One of the primary chemical reactions involving HCOOCH CH₂ H₂O is its hydrolysis, which is the process of breaking down the compound using water. In acidic or basic conditions, HCOOCH CH₂ H₂O undergoes hydrolysis to yield formic acid (HCOOH) and methanol (CH₃OH). This reaction is significant because both formic acid and methanol are highly useful in various industrial processes.
The reaction proceeds as follows:
HCOOCH CH₂ H₂O → HCOOH + CH₃OH
Formic acid, produced in this reaction, is widely used in manufacturing and industrial applications, such as in the production of plastics, textiles, and leather. Methanol, on the other hand, is a crucial solvent, fuel, and intermediate in the synthesis of other chemicals.
The hydrolysis of HCOOCH CH₂ H₂O is an essential reaction for obtaining these valuable chemicals, contributing to numerous sectors ranging from pharmaceuticals to agriculture.
In addition to hydrolysis, HCOOCH CH₂ H₂O can also participate in esterification reactions. Esterification is the process by which an ester is formed from an alcohol and an acid. In the case of HCOOCH CH₂ H₂O, the compound reacts with alcohols to form new esters, which can be used in a variety of applications, including fragrances, flavorings, and plasticizers.
Esterification reactions are crucial in the production of materials such as paints, coatings, and adhesives. The esters produced from HCOOCH CH₂ H₂O are often chosen for their stability and effectiveness in such applications.
Industrial Applications of HCOOCH CH₂ H₂O
The unique chemical properties of HCOOCH CH₂ H₂O make it highly valuable in several industrial applications. Below are a few industries where this compound plays a key role:
1. Solvent in Chemical Processes
One of the main uses of HCOOCH CH₂ H₂O is as a solvent in chemical processes. Due to its polarity and ability to dissolve a wide range of substances, this compound serves as an effective solvent in various chemical syntheses. It is especially useful in the pharmaceutical and chemical industries, where it helps dissolve and stabilize reagents and catalysts during the production of other chemicals.
2. Intermediate in Polymer Synthesis
In polymer chemistry, HCOOCH CH₂ H₂O acts as an intermediate in the production of synthetic polymers. Polymers are materials made from repeating structural units, and the use of formate esters in polymerization allows the creation of materials with desirable properties such as flexibility, durability, and resistance to environmental conditions. These materials are used in a wide range of industries, from automotive manufacturing to electronics.
3. Agricultural Applications
The derivatives of HCOOCH CH₂ H₂O are also used as preservatives in agriculture. These derivatives help extend the shelf life of fruits, vegetables, and other perishable goods by preventing microbial growth and oxidation. The compound is particularly beneficial in post-harvest handling, where it aids in maintaining the quality and freshness of produce during transportation and storage.
Environmental and Safety Considerations
While HCOOCH CH₂ H₂O is beneficial in many applications, it is essential to consider its environmental impact and safety. Proper handling and disposal are critical to preventing harm to the environment and human health.
1. Environmental Impact
Improper disposal of HCOOCH CH₂ H₂O and its derivatives can lead to contamination of water sources. These compounds can have a negative impact on aquatic life if not managed correctly. Therefore, proper waste disposal and treatment methods must be employed to prevent environmental damage. Sustainable practices, such as recycling and reducing waste, are essential to mitigate the potential environmental risks associated with this compound.
2. Safety Measures
HCOOCH CH₂ H₂O is flammable and should be handled with care. Inhalation or ingestion of the compound can be harmful, so appropriate safety gear such as gloves, goggles, and respirators should be used when working with it. Additionally, proper ventilation in workspaces where this compound is used can help prevent exposure to harmful fumes.
By adhering to safety guidelines and environmental protocols, the risks associated with HCOOCH CH₂ H₂O can be minimized while still reaping the benefits of its versatile applications.
Future Prospects and Research Directions
As organic chemistry continues to evolve, researchers are exploring new and more efficient ways to synthesize and use compounds like HCOOCH CH₂ H₂O. Current research focuses on improving the environmental sustainability of its production processes and expanding its applications in emerging industries.
1. Green Chemistry Initiatives
The field of green chemistry is dedicated to developing chemical processes that are environmentally friendly and reduce the use of hazardous materials. Researchers are looking for ways to synthesize HCOOCH CH₂ H₂O using renewable resources, which would make its production more sustainable and less harmful to the environment.
2. Advanced Material Development
The compound’s role in polymer synthesis is also being studied to create advanced materials with enhanced properties. These materials could be used in applications ranging from medical devices to space exploration, where the need for lightweight, durable, and flexible materials is crucial.
Research into the use of HCOOCH CH₂ H₂O for novel applications promises to unlock even more potential for this compound in the years to come.
Conclusion
HCOOCH CH₂ H₂O is more than just a chemical formula; it is a testament to the complexity and versatility of organic compounds. From its foundational role in chemical reactions to its diverse industrial applications, this compound exemplifies the dynamic nature of chemistry. As research continues to uncover its potential, HCOOCH CH₂ H₂O stands as a symbol of innovation and adaptability in the scientific world.